Diagnosing lead-acid battery faults
Servicing batteries
In use, a battery requires very little attention other than the following when necessary:
- Clean corrosion from terminals using hot water.
- Terminals should be smeared with petroleum jelly or Vaseline, not ordinary grease.
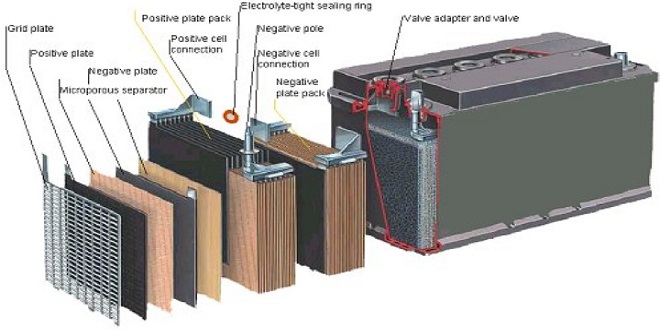
Battery faults
Any electrical device can suffer from two main faults; these are either open circuit or short circuit. A battery is no exception but can also suffer from other problems, such as low charge or low capacity. Often a problem – apparently with the vehicle battery – can be traced to another part of the vehicle such as the charging system
Repairing modern batteries is not possible. Most of the problems listed will require the battery to be replaced. In the case of sulphation it is sometimes possible to bring the battery back to life with a very long low current charge. A fortieth of the amperehour capacity or about a 1/200 of the cold start performance, for about 50 hours, is an appropriate rate
Testing batteries
For testing the state of charge of a non-sealed type of battery, a hydrometer can be used, as shown in Figure 5.6. The hydrometer comprises a syringe that draws electrolyte from a cell, and a float that will float at a particular depth in the electrolyte according to its density. The density or specific gravity is then read from the graduated scale on the float. A fully charged cell should show 1.280, 1.200 when half charged and 1.130 if discharged.
Most vehicles are now fitted with maintenancefree batteries and a hydrometer cannot be used to find the state of charge. This can only be determined from the voltage of the battery, as given in Table 5.3. An accurate voltmeter is required for this test. A heavy-duty (HD) discharge tester as shown in Figure 5.7 is an instrument consisting of a low-value resistor and a voltmeter connected to a pair of heavy test prods. The test prods are firmly pressed on to the battery terminals. The voltmeter reads the voltage of the battery on heavy discharge of 200–300 A.
Advanced battery technology
Electrochemistry
Electrochemistry is a very complex and wide-ranging science. This section is intended only to scratch the surface by introducing important terms and concepts. These will be helpful with the understanding of vehicle battery operation.
The branch of electrochemistry of interest here is the study of galvanic cells and electrolysis. When an electric current is passed through an electrolyte it causes certain chemical reactions and a migration of material. Some chemical reactions, when carried out under certain conditions will produce electrical energy at the expense of the free energy in the system.
Electrolytic conduction
Electricity flows through conductors in one of two ways. The first is by electron movement, as is the case with most metals. The other type of flow is by ionic movement, which may be charged atoms or molecules. For electricity to flow through an electrolyte, ion flow is required.
To explain electrolytic conduction, which is current flow through a liquid, sulphuric acid (H2SO4) is the best electrolyte example to choose. When in an aqueous solution (mixed with water), sulphuric acid dissociates into H, H and SO4, which are positive and negative ions. The positive charges are attracted to the negative electrode and the negative charges are attracted to the positive electrode. This movement is known as ion flow or ion drift.
Ohm’s Law and electrolytic resistance
The resistance of any substance depends on the following variables:
- Nature of the material.
- Temperature.
- Length.
- Cross-sectional area.
This is true for an electrolyte as well as solid conductors. Length and cross-sectional area have straightforward effects on the resistance of a sample, be it a solid or a liquid. Unlike most metals however, which have a positive temperature coefficient, electrolytes are generally the opposite and have a negative temperature coefficient.
Electrochemical action of the lead-acid battery
A fully charged lead-acid battery consists of lead peroxide (PbO2) as the positive plates, spongy lead (Pb) as the negative plates and diluted sulphuric acid (H2SO4) (H2O). The dilution of the electrolyte is at a relative density of 1.28. The lead is known as the active material and, in its two forms, has different valences. This means a different number of electrons exists in the outer shell of the pure lead than when present as a compound with oxygen.
Last word
The high temperature systems have, however, proved their viability for use in vehicles. They have already passed a series of abuse tests and other systems are in preparation. A sodium-sulphur battery when fully charged, which is rated at 20 kWh, contains about 10 kg of liquid sodium. Given 100 000 vehicles, 1000 tonnes of liquid sodium will be in use. These quantities have to be encapsulated in two hermetically sealed containers. The Swing concept is still new but offers a potentially safe system for use in the future.




